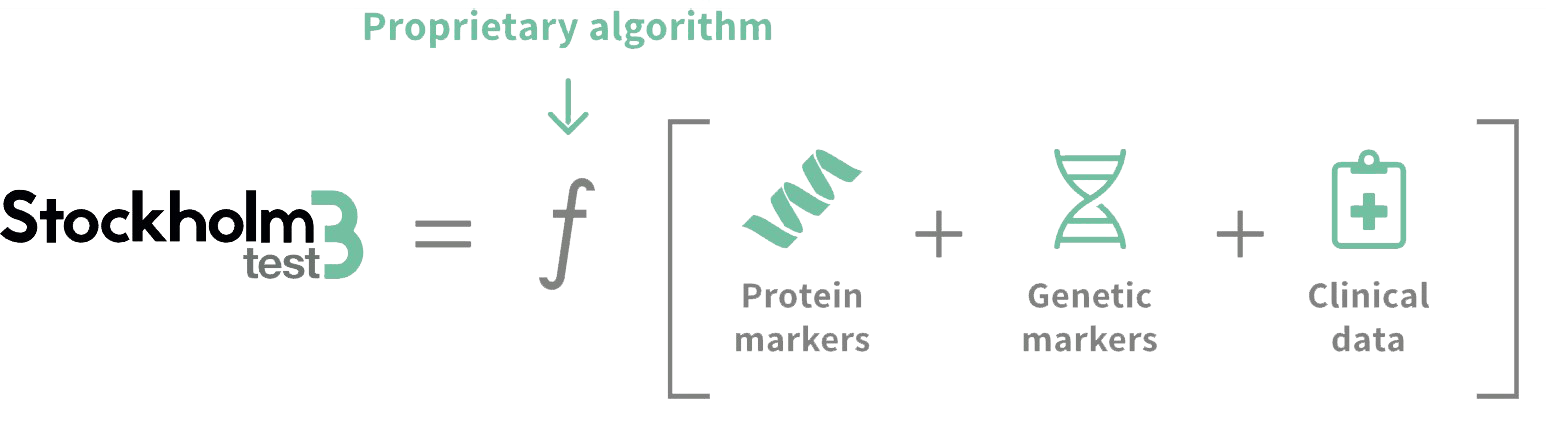
Stockholm3 is a blood test for early detection of aggressive prostate cancer. The test combines protein markers, genetic markers and clinical data in a proprietary algorithm for the purpose of detecting aggressive prostate cancer at an early stage. The result from Stockholm3 is a risk score that indicates the risk of aggressive prostate cancer and a recommendation that describes continued treatment.
A more precise test
Early detection is the key to successful treatment of prostate cancer. Today the blood test prostate-specific antigen (PSA) is used. One problem with PSA is that it misses between 30 and 50 percent of all aggressive cases of cancer. Research shows that Stockholm3 finds 100 percent more aggressive cases of cancer. This improves the ability to detect and treat cancer at an early stage.
Another problem with PSA is that it cannot distinguish between aggressive and benign cancer. Stockholm3 reduces the risk of conducting unnecessary prostate biopsies by 50 percent. As a result, many men do not have to undergo unnecessary follow-ups with a biopsy of the prostate, that can cause side effects such as sepsis as well as blood in the urine and feces.
Reduced costs
Healthcare providers in Sweden and Norway, who have replaced PSA with Stockholm3, show that direct care costs are reduced by 17 to 28 percent. This is because Stockholm3 reduces unnecessary and costly MRI’s, biopsies and treatments.
Enables population-based screening
Stockholm3 has been tested for general screening of prostate cancer. Stockholm3, in combination with MRI, reduces the number of unnecessary biopsies by 76 percent compared with PSA. In addition, the need for MRI is reduced by 60 percent and overdiagnosis is reduced by 39 percent. This makes the introduction of general screening of prostate cancer possible.
Developing Stockholm3
Stockholm3 has been developed by researchers at Karolinska Instituet. In total, data from more than 75,000 men has been included in studies of Stockholm3. The results have been published in highly ranked scientific journals such as The Lancet Oncology and European Urology. Stockholm3 has been available for clinical use since 2017. Several healthcare providers in Europe are now using Stockholm3. The company is ISO 13485 certified, and the software complies with IEC 62304 standard for medical technology software.
Test result
The result from Stockholm3 is a risk score that indicates the risk of aggressive prostate cancer, elevated risk, normal risk or low risk. Stockholm3 also provides clear treatment recommendations which makes it easier for the treating doctor to make a decision on potential further actions. It also helps the man to get a better understanding of his individual risk of getting prostate cancer. With elevated risk, a referral to a urologist for further examination is recommended, and with low or normal risk a new test within two to six years is recommended. Nearly half of the men aged 50 to 70 years have a low risk profile and do not need to take a new test until after six years.
The intended target group for Stockholm3 is men between the ages of 45 and 74.
